Palladium sputtering targets have common characteristics with their raw materials. Palladium, together with rhodium, ruthenium, osmium, iridium, and platinum form a group of elements referred to as the platinum group metals. Palladium is a soft silver-white metal that resembles platinum. It is the least dense and has the lowest melting point of the platinum group metals. It is soft and ductile when annealed and is greatly increased in strength and hardness when cold-worked. It has a face-centered cubic crystalline structure, at ordinary temperatures it is strongly resistant to corrosion in air and to the action of acids. It is attacked by hot acids, and it dissolves in aqua regia. It forms many compounds and several complex salts. Palladium has a great ability to absorb hydrogen (up to 900 times its own volume).
Because of its corrosion resistance, a major use of palladium is in alloys used in low voltage electrical contacts. When it is finely divided, palladium forms a good catalyst and is used to speed up hydrogenation and dehydrogenation reactions.
Palladium is used extensively in jewelry-making in certain alloys called “white gold.” It may be alloyed with platinum or substituted for it. It is used in watch bearings, springs, and balance wheels and also for mirrors in scientific instruments.



.png)
 +86-731-84027969
+86-731-84027969
 info@xk-sputteringtarget.com
info@xk-sputteringtarget.com


.png)
.png)




.png)

.png)
.png)

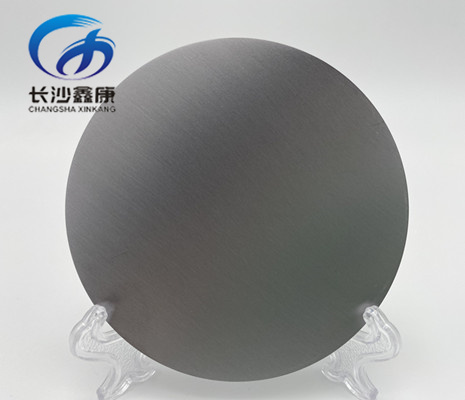
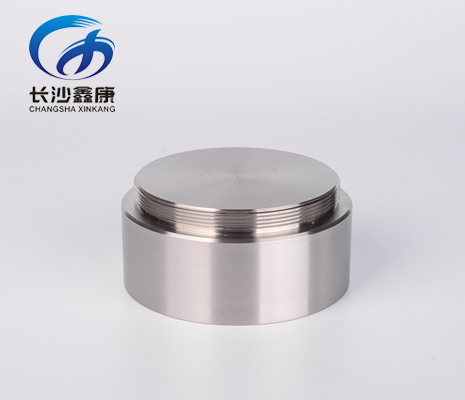

.png)